Before starting any troubleshooting, whether it is related to instruments or columns, it is essential that safe laboratory practices be observed. The chemical and physical properties of any solvents used should be known and the material safety data sheet (MSDS) for theses solvents should be readily available.
All electrically powered instruments should be powered down and unplugged
from the main supply before the removal of their covers, etc. Wear eye protection when troubleshooting the detector (with the cover removed) as ultraviolet light is emitted during UV and fluorescence detector operation and this will damage the eye’s cornea irreversibly.
There are many areas in a HPLC instrument that give rise to system and chromatographic problems. This guide will deal with each one in the following
sections:
- Visual Inspection
- Pressure
- Baseline Irregularities
- Changes in Chromatography
- Qualitative Results
- Quantitative Results
1. Visual Inspection
- When a problem occurs, it is advisable to perform a quick visual check of the instrument and column. This will pick up leaks, loose or disconnected tubing, changes in instrument settings, etc…
2. Pressure
- System pressure is affected by a number of variables including the viscosity of the solvent used, column variables, flow rate and temperature. It is important to have a reference point when high or low pressures to the norm. This reference point should be the pressure generated in the system when everything if functioning correctly.
- Pressure problems fall into one of three categories: high, low or fluctuating pressure. They can occur suddenly or be a gradual process. Sudden pressure rises tend to be due to particles from the sample, blocked or damaged tubing or column packed bed collapse. Gradual pressure rises can also be due to particles in the sample, but they can also arise from particles generated in the instrument, for example, debris from vial septa or degrading seals.
- Before releasing any high pressure build-up in a system, be aware that the solvent may form an aerosol or spray when loosening connections. Eye protection should be worn and ideally the connection to be positioned above and adsorbent material to soak up all released solvents.
The simplest way to troubleshoot pressure problems is using a systematic approach, as highlighted in the following tables for high, low or fluctuating pressure.
2.1. High pressure
- The most common causes of high pressure are blocked tubing around the injector and column inlet.
2.2. Low pressure
- The most common causes of no/low pressure are the solvent inlet lines not being immersed in solvent, no solvent in the reservoir and leaks.
3.3. Fluctuating pressure
- The most common cause of fluctuating pressure is poorly primed lines with badly degassed solvents.
ALSO READ: SOP for Preventive Maintenance of HPLC
3. Baseline Irregularities
- Baseline irregularities can be non-cyclic or cyclic. They can originate from electrical interferences, detector faults, solvent impurities, column contamination, etc.
- To isolate the source of a baseline irregularity, it is important to determine whether the problem lies with the fluid path, detector or electrical connections. This can be achieved by following the simple steps below:
- Turn off the instrument pump - fluid flow must be zero
- Monitor the baseline for 5 to 10 min. note if there is any improvement in the baseline’s appearance. If yes, then the problem lies within the instrument's fluid path. If no, the problem is either electrical or detector related.
- Disconnect the detector electrical cables from the A/D interface with PC, integrator and chart recorder, i.e. the data handling devices. Attach a jump source to the input terminals on the data-handling device (a crocodile clip, paper clip). If the noise continues, then the problem is within the data-handling device.
- Data-handling device troubleshooting is beyond the scope of this guide: Contact your instrument provider for this service.
- The sections provide a quick reference guide for typical baseline irregularities, their causes and corrective action that can be taken to cure the problem.
3.1. Non-cyclic noise – Fluid path problems
- The most common cause of non-cyclic baseline noise related problems is air in the system. To overcome this, all solvents should be thoroughly degassed prior to use, all lines should be purged with solvent and the pump, should be thoroughly primed.
- Air bubbles can obscure the detector flow cell and baseline noise – be aware that from time to time, the cell may require cleaning and/or removal of air bubbles.

3.2. Non-cyclic noise – Detector electronics problems
- The most common cause of problems related to electronic baseline noise is the detector. Usually, if the detector is allowed insufficient time to equilibrate before an injection is performed, then the resultant chromatogram will contain spurious peaks and there will also be some evidence of baseline drift.
- If the problem occurs after routine maintenance, check that all the cables are securely seated in their sockets and that the correct cable is in the correct socket.
- Also, check that all settings have been returned to their position prior to the routine maintenance.
3.3. Cyclic noise – Detector-related problems and others
4. Changes in chromatography
- The most common changes in chromatographic responses are related to the shape and separation of peaks, their elution times and changes in established performance.
- The evaluation stage is best preformed on a standard rather than sample injection. The nature and prior chromatographic performance of the standard should be recorded each time it is injected. This will provide historical data for any comparisons that you need to perform.
- The following sections will list each of the most common causes of change in chromatographic response and corrective action that can be made to return your chromatography to its previous state.
4.1. Retention time changes from injection to injection
- The most common cause of peak retention time drift is an un-equilibrated system. The detector and fluid system must be stable prior to starting an analysis.
- Temperature changes during the analysis are another major cause of peak drift. If your analytical column is subject to fluctuations in temperature, then we recommended that the column is housed in a thermally controlled environment, such as a column oven/jacket, etc.
We recommend the pre-mixing of all solvents used in isocratic methods.
4.2. Continually increasing or decreasing retention times
- The most common cause of peak retention time drift in one direction is poorly prepared or mixed solvents or a system leak. If you are confident that the solvents were prepared correctly, then it is very important that you determine whether they are being mixed correctly (mixing cell problems). Where solvents are mixed manually prior to pumping, ensure that the solvent flow rate is correct and constant.
4.3. Increasing/decreasing to a new constant retention time
- The last most common cause of retention time change is a leak in the system or build-up of contaminants.
4.4. Abnormal peak shape
- Abnormal peak shape encompasses a range of possible peak shape problems:
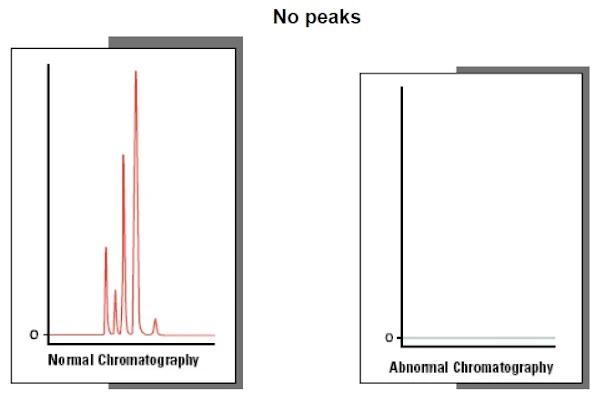
- No peaks
- Fronting or tailing peaks
- Smaller-than-expected peaks
- Double peaks/shouldering peaks
- Broad peaks – early eluting analytes or all analytes
- Flat-topped peaks
- Negative peaks
- If all peaks in a chromatogram are affected, then it suggests that the problem is related to either the system or the column. If only early eluting peaks are affected then it suggests that the problem lies within the fluid path – perhaps with incorrect ID tubing, fitting, etc.
- If single peaks are affected, then it suggests that there might be a specific chemistry problem. The method in use should be examined for areas where the chemistry may not be correct.
- Gradient methods, where early eluting peaks are abnormal and later peaks are acceptable may be suffering from pre-column band broadening. If all peaks are abnormal, then post-column band broadening or other changes in the system are the most likely causes.
- Isocratic methods, where early eluting peaks are abnormal and later peaks are acceptable may be suffering from extra-column band broadening, injector problems, incorrect detector time constant or incorrect A/D sampling time. If all peaks are abnormal, then extra-column band broadening or other changes in the system are the most likely causes.
- Each table in this section will show an example of the type of chromatography being investigated, it is cause and any corrective action that can be taken.
- Lack of chromatogram peaks is often due to either the wrong sample being injected, the detector not being switched on or a blockage between the injector and detector lines.
- The next most common reason for a lack of peaks is that some part of the sample or mobile phase preparation has been performed incorrectly, so it is always worth revisiting to check that the correct buffer has been used, the sample/solvent pH is correct, etc.
- Smaller than expected peaks are often due to either the wrong sample being injected, an incorrect sample volume being injected, or a blockage between the syringe needle and detector. Problems with the syringe plunger sticking in the barrel can occur if the sample contains particulates.
Note:
Viscous sample will require a longer draw time. Insufficient draw time will result in a lower volume of sample being injected onto the column and smaller peaks will result.
- Broad early eluting peaks are most commonly associated with sample overload or incorrect system plumbing.
All peaks broad
Broad peaks are most often due to errors in instrumentation or column. It is worthwhile investigating the column and guards first as they often are the critical part of the system.
The most common cause of peak doubling can be either blockage prior to the column or guard voiding.
Fronting peaks are very often due to large injection volumes of a sample that is dissolved in solvents that are incompatible with the mobile phase being used. The next most common cause of peak fronting is a voided or contaminated guard or column.
Tailing peaks
Tailing peaks are typically caused by column degradation or inlet contamination. Carefully maintained columns and guards will considerably reduce the incidence of tailing peaks.
Flat topped peaks
Flat-topped peaks are most often caused by either large injection volumes of dilute samples or by small injection volumes of strong sample dilution.
Negative peaks
Negative peaks are most often caused by differences in refractive index between the sample solvent, sample and mobile phase. They are also caused after routine maintenance when the system has not been reconfigured correctly.
5. Qualitative results
- Qualitative assays do not measure exact quantities of an analyte in solution. Therefore, problems associated with these assays usually fall into one of two categories:
a) missing or extra peaks and
b) peak misidentification.
- The following tables will assist in tracing errors in qualitative methods.
5.1. Missing peaks
- Single or multiple missing peaks are usually due to the wrong sample being injected of the sample degrading. Equally likely though is a loss of resolution due to column/solvent inconsistencies.
5.2. Extra peaks
- Extra peaks in chromatograms are more often than not due to contamination or degradation of the sample, mobile phase or column. To check if the extra peaks are in the sample alone, perform a blank injection of sample solvent. The peaks should be absent.
5.3. Peaks misidentified
- Peaks misidentification occurs most often in degradation samples or those in which related substance levels are being measured. This is because the software is “calibrated” using a standard mixture with specific concentrations of each impurity.
- Most “real-life” samples contain impurities at very low levels so the retention time of their peaks will be slightly different to those generated by the standard mix. Identification windows should be set widely enough to take into account this time variation with respect to concentration.
6. Quantitative results
- Quantitative assays measure exact quantities of an analyte in solution. Therefore, problems associated with these assays usually fall into one of two categories:
- Loss of precision
- Loss of accuracy.
- The most common mistake made is to assume that accuracy and precision are the same. Accuracy is defined as the proximity of a result to the true value whereas precision is a measure of reproducibility of the result. It is important to determine whether your method is inaccurate or imprecise, as this will determine the steps taken to rectify the problem. In general, is the result generated is correct, but reproducible then the problem is accuracy based. If the results generated vary, then the problem is precision-based.
The following tables will assist in tracing errors in quantitative methods.
6.1. Loss of accuracy
Loss of accuracy is most often related to the sample.
6.2. Loss of precision
- Loss of precision is most often caused by an injector error, by a sample that is mixed poorly, or a sample that is degrading.
ALSO READ: Common Mistakes to Avoid When Using HPLC Mobile Phases














































0 Comments